This infographic shows why the safety level used by the FDA is over 8x too high.
Sources:
What does the science say?
This infographic shows why the safety level used by the FDA is over 8x too high.
Sources:
Autism is defined behaviourally by the two characteristics: 1) Persistent deficits in social communication and social interaction across multiple contexts, and 2) Restricted, repetitive patterns of behavior, interests, or activities. This definition comes from DSM-5 (2013), which classifies autism as a neurodevelopmental disorder, a type of mental disorder. There are three levels of severity on each characteristic, based on the extent of support required.
Something is causing children’s brains to be altered during neurodevelopment, resulting in autistic behaviours. Shouldn’t we try and find out what is causing that? An investigation into cause does not require any judgement about whether more autistic children is “good” or “bad”, or whether being autistic is a strength or a weakness. It is merely an attempt to explain the observation that autism occurs and is increasing.
The number of people diagnosed with autism has increased dramatically since 1985, from 1-in-10000 to 1-in-35, and the number is still increasing rapidly (Nevison, 2014). This dramatic rise cannot be explained by changes to the definition of autism or to improved diagnosis and awareness. Something is causing more children to develop autism. The increase is real, not technical (Nevison, 2017).
The increase is too rapid to be explained as genetic, so the cause must be environmental. We know from twin studies that autism is partly genetic and partly caused by environmental factors (Hallmayer, 2011).
Autism has been linked to multiple environmental toxins, including glyphosate, electro-magnetic frequencies, and vaccine ingredients such as aluminum and thimerasol (Mercola, 2018). Environmental toxins activate the innate immune system and initiate the inflammatory process. Inflammation is excessive in the brains of autistic people, demonstrating neurotoxicity (Vargas, 2005 and Tsilioni, 2019).
Maternal immune activation (MIA) has been identified as a cause of autism, through both epidemiological and animal studies. A severe infection during pregnancy is associated with autism (Zerbo, 2015). Autistic behaviours can be induced in animals by activating the immune system during pregnancy (Malkova, 2012). Post-natal immune activation has also been found to cause autistic behaviours in animals (Missig, 2018).
The particular toxin or pathogen appears to be less important than the strength and timing of the immune activation during early neurodevelopment. This is why any or all of the above-mentioned environmental toxins could be a cause of autism. Since the focus of this website is vaccines in general, and aluminum toxicity in particular, I will explain specifically why aluminum in vaccines is a likely cause of autism.
Aluminum toxicity has been recognised as a major cause of encephalopathy (brain damage) in uremic patients, whose kidneys are less effective at eliminating aluminum (Alfrey, 1993).
Aluminum can have more subtle neurological effects, only detected through behavioural tests, even when the kidneys are healthy. Subtle neurological effects have been observed in workers chronically exposed to aluminum dust or fumes (ATSDR, 2008). Preterm babies exposed to high levels of aluminum through Total Parenteral Nutrition (TPN) scored lower in mental development tests (Bishop 1997), leading the FDA to place a limit on aluminum content in TPN products (FDA, 21CFR201.323).
In animal models, aluminum at sufficient doses in the diet induces: neuroinflammation (Viezeliene, 2013); loss of neuronal dendritic spine and cognition impairment (Cao, 2016); memory impairment (Martinez, 2017); adverse reproductive effects (Hirata-Koizumi, 2011); learning delay (Bilkei-Gorzo, 1993); decreased limb strength (Poirier, 2011); and decreased motor performance (Golub, 2001).
Based on these animal studies, health agencies such as the WHO (JECFA, 2011) have established safety limits for oral intake of aluminum. The current WHO oral intake safety limit is 0.3 mg/kgbw/day.
High levels of aluminum have been observed in the brains of individuals who had neurological conditions, including Alzheimer’s disease (Mirza, 2017) and autism (Mold, 2018). A meta-analysis showed that excessive aluminum in blood, urine, and hair is associated with autism (Sulaiman, 2020).
Given all this, we ought to be deeply concerned with the possibility that aluminum toxicity may be altering the neurodevelopment of children, resulting in autism.
The primary source of exposure to aluminum during infancy is from vaccines. Exposure through diet and air is limited by very low permeability of the gut and lung linings to aluminum. The aluminum in vaccines on the other hand all enters systemic circulation, since it has no other way of leaving the injection site. Over the whole of infancy, uptake of aluminum from vaccines is around 50x higher than uptake from breast milk and air, as I demonstrated here.
The WHO oral intake safety level of 0.3 mg/kgbw/day can be converted into an uptake safety level by multiplying the oral intake safety level by the gut bioavailability of ingested aluminum. When this is done, it can be seen that aluminum uptake from vaccines exceeds the implied WHO uptake safety level, as I demonstrated here.
The only evidence cited by the CDC that the amount of aluminum in infant vaccines is safe is a pharmacokinetic model (Mitkus, 2011) which has many critical errors and weaknesses; I wrote about one of them here.
No large-scale epidemiological studies have been carried out looking for an association between aluminum-containing vaccines and autism (IOM, 2012). The vaccine schedule as a whole has not been tested (IOM, 2013)
This is despite the existence of an extensive database called the Vaccine Safety Datalink (VSD), controlled by the CDC. The Institute of Medicine described the VSD as the most feasible way to conduct large scale-studies comparing health outcomes of vaccinated and unvaccinated children (IOM, 2013), but still no such studies have been done.
Some independently-funded small-scale epidemiological studies have been conducted:
Finally, mice vaccinated to mimic the CDC recommended schedule were found to exhibit autistic behaviour (Sheth, 2018).
Given this abundance of evidence implicating aluminum in vaccines as a likely cause of autism, it is urgent that large-scale epidemiological studies are carried out to test this highly-plausible hypothesis. Parents need to be informed about the evidence that the aluminum in vaccines can alter the neurodevelopment of their children.
According to the Agency for Toxic Substances and Disease Registry (ATSDR), from their Toxicological Profile of Aluminum (2008), aluminum has subtle neurological effects detected with neurobehavioural performance tests…
Neurodegenerative changes in the brain, manifested as intraneuronal hyperphosphorylated neurofilamentous aggregates, is a characteristic response to aluminum in certain species and nonnatural exposure situations generally involving direct application to brain tissue, particularly intracerebral and intracisternal administration and in vitro incubation in rabbits, cats, ferrets, and nonhuman primates.
Oral studies in rats and mice have not found significant histopathological changes in the brain under typical exposure conditions; however, altered myelination was found in the spinal cord of mouse pups exposed to 330 mg Al/kg/day on gestation day 1 through postnatal day 35.
Overt signs of neurotoxicity are rarely reported at the doses tested in the available animal studies ( ≤330mg Al/kg/day for bioavailable aluminum compounds); rather, exposure to these doses is associated with subtle neurological effects detected with neurobehavioral performance tests.
Significant alterations in motor function, sensory function, and cognitive function have been detected following exposure to adult or weanling rats and mice or following gestation and/or lactation exposure of rats and mice to aluminum lactate, aluminum nitrate, and aluminum chloride. The most consistently affected performance tests were forelimb and/or hindlimb grip strength, spontaneous motor activity, thermal sensitivity, and startle responsiveness.
Significant impairments in cognitive function have been observed in some studies, although this has not been found in other studies even at higher doses. Adverse neurological effects have been observed in rats and mice at doses of 100–200 mg Al/kg/day and neurodevelopmental effects have been observed in rats and mice at doses of 103–330 mg Al/kg/day.
ATSDR 2008
The Flarend 1997 paper is used for the absorption part of the Mitkus pharmacokinetic model, on which the CDC relies for their claim that aluminum in vaccines is safe.
This infographic describes the experiment and its weaknesses. It also shows that the outputs of the Mitkus model contradict the excretion results from the Flarend experiment.
Here I explain the simple calculation that shows why the amount of aluminum in vaccines exceeds the WHO safety level.
Aluminum is a neurotoxin; at high enough doses, the toxic effects of aluminum show up primarily in the brain.
This is why the FDA set a limit of 25 mcg/l of aluminum in Total Parenteral Nutrition (TPN) products. The limit was set based on a 1997 study of premature neonates. Those fed by TPN (for 10-20 days) with a high-aluminum product scored lower on the Bayley Mental Development Index than those fed a low-aluminum alternative.
Most of our knowledge about the dose-response effects of chronic aluminum exposure comes from animal studies. Aluminum is added to food or drinking water and the animals are tested for adverse events, primarily neurodevelopmental.
Based on a survey of these animal studies, the WHO Joint Expert Committee on Food Additives (JECFA) established a Provisional Tolerale Weekly Intake (PTWI) level for humans. Their 2011 report set the oral intake safety level at 2 mg/kgbw (having previously been 1 mg/kgbw, and before that 7 mg/kgbw).
There is no safety level for aluminum in vaccines. The FDA sets a limit of 850mcg per vaccine dose, but this limit is based on efficacy, not safety. A safety level for aluminum in vaccines would need to consider that multiple doses of different vaccines are given simultaneously and doses are repeated in close succession in infancy.
In the absence of a safety level for aluminum in vaccines, the best we can do is use the WHO / JECFA oral intake safety level as a guide. We know that gut bioavailablility of aluminum is only 0.1%-0.3%. A diet at the JECFA oral safety level contains 2000 mcg/kgbw per week, or 300 mcg/kgbw/day. So that equates to 0.3-0.9 mcg/kgbw/day entering the blood.
We can take the top end of this range and call this the WHO / JECFA uptake safety level. If there is more than 0.9 mcg/kgbw of aluminum entering the bloodstream each day, there is a risk of adverse neurological effects. For an average-weight male infant, this equates to 3.2 mcg at birth, up to 9.2 mcg at 12 months old.
Using the CDC schedule, the total amount of aluminum in vaccine doses given in the first six months of infancy is 3,450 mcg. The largest single amount of 1,225 mcg given at 2 months old. The DTaP alone contains 625 mcg, and is given at 2, 4, and 6 months old.
In my previous post, I explained the calculation of aluminum exposure to infants by source. The infographic below shows that when bioavailability is factored in, vaccines are by far the biggest source of aluminum exposure in infants: up to 98%.
Vaccines are administed intramuscularly; they do not enter the bloodstream immediately. The aluminum is in the form of a salt, usually aluminum hydroxide. It is believed that all the aluminum salt dissolves slowly at the injection site. Once dissolved, the aluminum ions bind to transferrin in the bloodstream. Eventually, all the aluminum in the vaccine enters the bloodstream.
To calculate how much aluminum enters the bloodstream each day from vaccine injection sites, we first assume that all the aluminum dissolves (as opposed to being carried away by macrophages). Then we must estimate the rate and order of the absorption process. We have two animal studies that we can reference for this. Flarend (1997) calculated that 17% of aluminum hydroxide was absorbed in the first 28 days after IM administration. Weisser (2019) calculated that 22% was absorbed in the first 80 days.
Assuming a zero-order process, these results mean 0.6% (Flarend) or 0.3% (Weisser) is absorbed per day. Full absorption would then take 165 days (Flarend) or 369 days (Weisser). Flarend and Weisser both found that aluminum phosphate (used in the Pneumococcal vaccine) is absorbed about 3x faster than aluminum hydroxide.
Based on the absorption data from Weisser, we can plot how much aluminum enters an infant’s bloodstream each day from their vaccine injection sites. We can compare it to the WHO / JECFA safety level. In the chart, the safety level for an average-weight male infant is shown. Uptake from diet (breast milk for six months, followed by semisolid food of average aluminum content) and air is also shown for comparison.
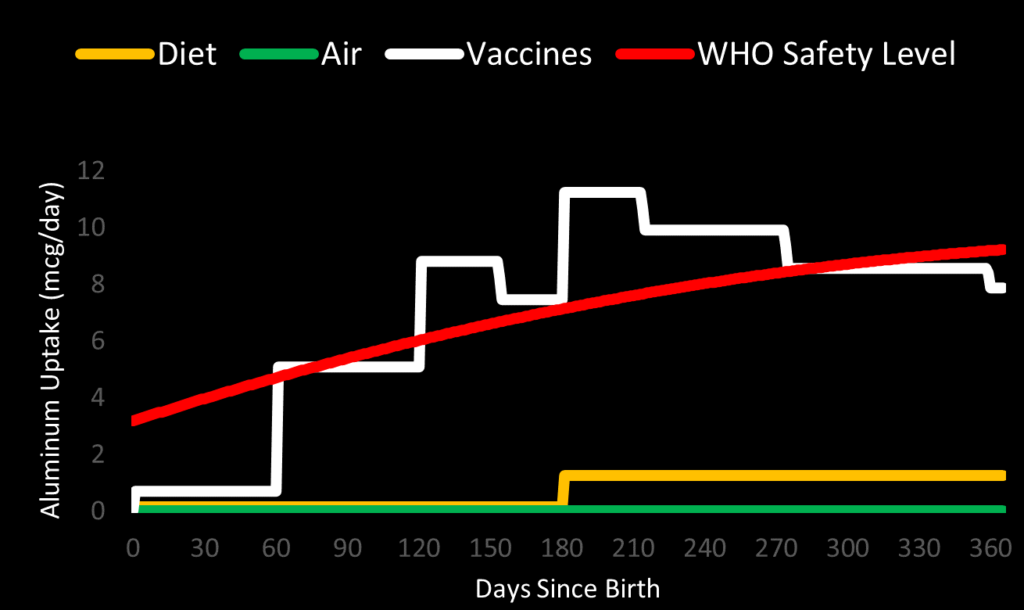
We can see that daily aluminum uptake jumps up to 5.1 mcg/day after the 2-month shots, exceeding the safety level. It peaks at 11.2 mcg/day after the 6-month shots, when aluminum is entering the blood from six previous vaccine shots. For most of infancy, the uptake from vaccines alone exceeds the WHO / JECFA safety level. It is considerably worse for underweight infants.
All vaccinated infants are being exposed to aluminum at doses that risk adverse neurological effcts. This is an urgent safety issue that is being ignored by the authorities responsible for vaccine safety.
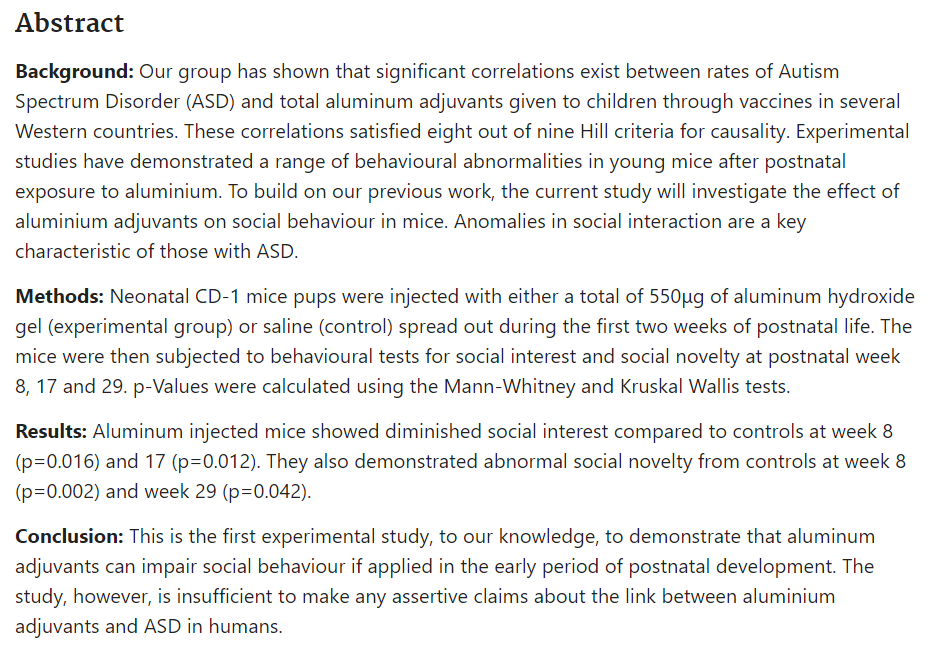
Baby mice injected with aluminum adjuvants to mimic the CDC vaccination schedule scored lower on tests of social interaction.
Source:
Is exposure to aluminium adjuvants associated with social impairments in mice? A pilot study, Sheth 2018, https://pubmed.ncbi.nlm.nih.gov/29221615/
This post is my review of the CDC webpage titled Adjuvants and Vaccines.
The CDC writes:
An adjuvant is an ingredient used in some vaccines that helps create a stronger immune response in people receiving the vaccine. In other words, adjuvants help vaccines work better. Some vaccines that are made from weakened or killed germs contain naturally occurring adjuvants and help the body produce a strong protective immune response. However, most vaccines developed today include just small components of germs, such as their proteins, rather than the entire virus or bacteria. Adjuvants help the body to produce an immune response strong enough to protect the person from the disease he or she is being vaccinated against. Adjuvanted vaccines can cause more local reactions (such as redness, swelling, and pain at the injection site) and more systemic reactions (such as fever, chills and body aches) than non-adjuvanted vaccines.
By design then, vaccine adjuvants are necessarily toxic. This is because their purpose is to induce an immune response stronger than the immune response to the antigen component alone. The adjuvant is what makes the challenge to the immune system from the vaccine strong enough to induce immunological memory.
This is why the safety of vaccine adjuvants is so important to the safety of vaccines as a whole; the adjuvant is by design the most toxic part of the vaccine.
The CDC writes that:
Aluminum salts, such as aluminum hydroxide, aluminum phosphate, and aluminum potassium sulfate have been used safely in vaccines for more than 70 years. Aluminum salts were initially used in the 1930s, 1940s, and 1950s with diphtheria and tetanus vaccines after it was found they strengthened the body’s immune response to these vaccines.
Aluminum salts have indeed been used since the 1930s, as described in the earliest papers about aluminum adjuvants, such as Glenny 1926 and Volk 1942. Whether they have been used safely is the question at hand.
There were no safety studies done on the effects of Aluminum-Containing Vaccines (ACVs) prior to their routine use in infants. The only studies were of efficacy, tested by measuring antibodies. The first routine ACVs were the diphtheria, tetanus, and pertussis vaccines. These were later given as a combined vaccine, known as DTP, and then DTaP (the ‘a’ referring to acellular pertussis).
Up until 1990, the DTP vaccine was the only ACV given routinely to infants. After 1990 however, the number of ACVs on the recommended infant vaccine schedule started increasing. The Hib and Hepatitis B vaccines were added in 1991 and the Pneumococcal vaccine in 2000. They started being given in more doses and at a younger age. Infants today are considerably more exposed to aluminum from vaccines than infants of 30 years ago.
Aluminum is classified by the US Agency for Toxic Substances and Disease Registry (ATSDR) as a neurotoxin. This means the first harmful effects of aluminum overdose occur in the brain. The ATSDR describe aluminum as having “subtle neurological effects”, even at low doses. A steady increase in neurodevelopmental disorders, e.g. ASD, ADHD, and intellectual disability, is to be expected if aluminum adjuvants in infant vaccines are altering neurodevelopment.
We have seen an increase in neurodevelopmental disorders in line with increasing exposure to aluminum from vaccines. The increase cannot be explained away as expanded definitions, better diagnosis, or increased awareness. This ecological observation – the temporal correlation between ACV use and neurological disorders – calls for further research. As a neurotoxin, aluminum has to be a prime suspect in the search for causes of neurodevelopmental disorders.
The CDC writes that:
Newer adjuvants have been developed to target specific components of the body’s immune response, so that protection against disease is stronger and lasts longer.
None of the newer adjuvants are yet used in infant or childhood vaccines. They are thus of no relevance to an investigation of adjuvant safety in the infant and childhood vaccine schedule. The safety of adjuvants in this context means the safety of aluminum salts.
The CDC writes that:
In all cases, vaccines containing adjuvants are tested for safety and effectiveness in clinical trials before they are licensed for use in the United States, and they are continuously monitored by CDC and FDA once they are approved.
In pre-approval clinical trials, potential new vaccines are tested against a placebo injection. The primary focus of the trials is efficacy; a vaccine is judged as successful when it results in the development of antibodies. Safety is a secondary consideration. Typically, immediate adverse reactions are recorded, there is active follow-up for only a week, and passive follow-up for only a year.
Pre-approval clinical trials will not pick up long-term adverse reactions, chronic conditions or altered neurodevelopment. No rare adverse events are picked up in the clinical trials, due to the small sample size.
For assessing adjuvant safety, pre-approval clinical trials are entirely useless. This is because both the vaccine being tested and the placebo contain adjuvant! The placebo is either a solution of adjuvant only, or an already-licensed vaccine. There are no safety tests of vaccine adjuvants in pre-approval clinical trials of ACVs.
The post-approval monitoring systems of the CDC are the Vaccine Adverse Event Reporting System (VAERS) and the Vaccine Safety Datalink (VSD). VAERS is a system is for reporting immediate and short-term vaccine reactions. It cannot be used to monitor, for example, whether ACVs are causing autism. The VSD is a database that could easily and cheaply be used to conduct large-scale epidemiological studies. It is a data goldmine.
The Institute of Medicine, in their 2013 report “The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies” recommended using the VSD to assess vaccine safety:
The most feasible approach to studying the safety of the childhood immunization schedule is through analyses of data obtained by VSD. VSD is a collaborative effort between CDC and 9 managed care organizations that maintain a large database of linked data for monitoring immunization safety and studying potential rare and serious adverse events. VSD member sites include data for more than 9 million children and adults receiving vaccinations on a variety of immunization schedules.
No studies have been on VSD data looking for a possible association between ACVs and neurodevelopmental conditions. The IOM’s call for more safety studies of the vaccine schedule has gone unheeded, so far.
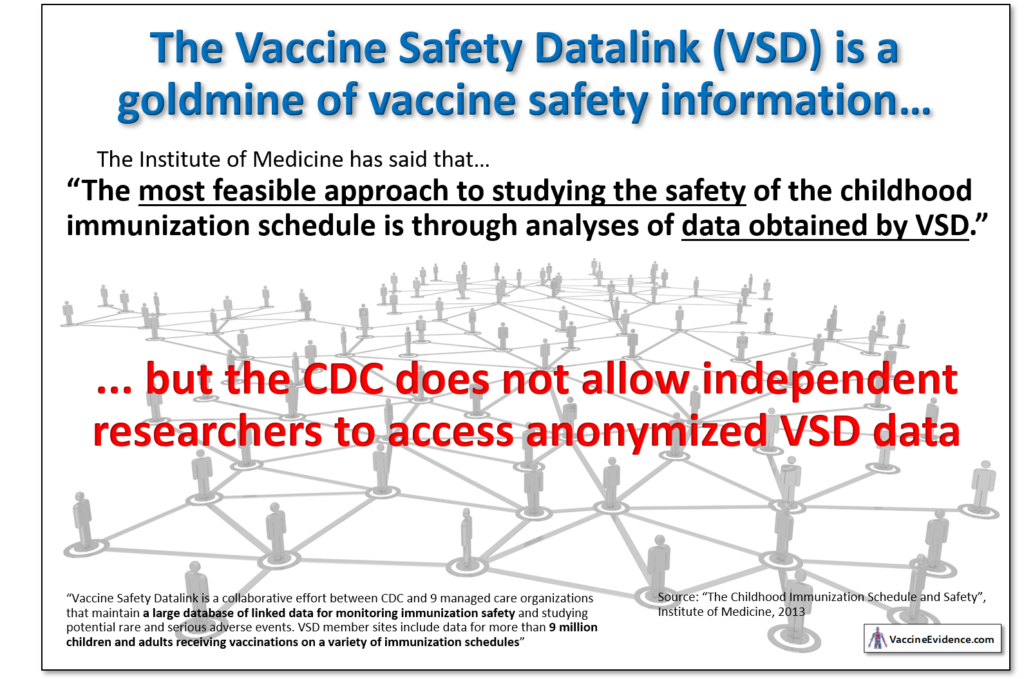
The VSD has great potential to increase vaccine take-up by allaying many concerns people about vaccine safety. For reasons unknown, the CDC refuses to allow researchers to access the VSD data to perform these kinds of studies. Nor has it produced any studies of its own using the VSD.
The CDC provides a table showing which vaccines use aluminum-based adjuvants, other adjuvants, or no adjuvant.

Four different aluminum salt forms are listed in the table: AAHS, AH, AP and Alum. Three of these are in vaccines on the CDC’s infant vaccination schedule.
The following table shows the ACVs on the CDC’s infant vaccine schedule, with the most popular brands chosen where applicable. The aluminum-weight in each dose is given, along with the aluminum salt form they use, and the ages at which doses are given.
| Vaccine | Brand | Al Content (mcg) | Adjuvant Form | Doses Given At (months) |
| HepB | Engerix-B | 250 | Aluminum Hydroxide | 0,2,6 |
| DTaP | Infanrix | 625 | Aluminum Hydroxide | 2,4,6 |
| Hib | PedvaxHIB | 225 | AAHS | 2,4,12 |
| PCV13 | Prevnar 13 | 125 | Aluminum Phosphate | 2,4,6,12 |
| HepA | Havrix | 500 | Aluminum Hydroxide | 12 |
The highest single day exposure is at 2 months old, when 1,225 mcg is injected at once. The total aluminum content of the vaccines given to infants in the first six months is 3,450 mcg (3.45 mg).
The CDC writes that:
Aluminum-containing adjuvants are vaccine ingredients that have been used in vaccines since the 1930s. Small amounts of aluminum are added to help the body build stronger immunity against the germ in the vaccine.
The term “small” requires some reference value. Is 3.45 mg in six months a “small” or “large” amount of exposure to aluminum for an infant?
One way to answer this question is to compare it to aluminum exposure from other sources.
The CDC writes that:
Aluminum is one of the most common metals found in nature and is present in air, food, and water.
We are indeed all exposed to aluminum in our food, drinking water, and air. By knowing the aluminum content of our food, drinking water, and air, we can estimate our total intake. Using estimates of gut and lung bioavailability, we can calculate our total uptake, i.e. how much aluminum is entering our blood.
The infographic below shows this calculation:

The details of this calculation and sources can be found in my post: Aluminum Uptake in Infants
It can be seen that for some infants, 98% of aluminum exposure over the year comes from their vaccines. If they are exclusively breastfed they will only uptake 50 mcg through their gut over the entire year. Exposure from city air of average quality is only 6 mcg. Even infants fed high-aluminum soy formula or grain-based baby foods will uptake no more than 2,000 mcg of aluminum through their gut. Still a lot less than the 3,450 mcg aluminum they received from their vaccines.
Since vaccines are the primary source of aluminum exposure to infants, it cannot be claimed that the amount of aluminum in vaccines is “small”.
The CDC writes that:
Scientific research has shown the amount of aluminum exposure in people who follow the recommended vaccine schedule is low and is not readily absorbed by the body.
The phrase “not readily absorbed by the body” is certainly true in the case of aluminum exposure in food, drinking water, and air. Gut bioavailability (the percentage of intake that enters the blood) is less than 0.3% and lung bioavailability is only 2.0%. Hardly any of the intake from diet and air enters the body; aluminum is not readily absorbed through the guts or lungs.
Vaccines are injected into muscle. There is no way for the aluminum in vaccines to leave the injection site except by entering the systemic circulation. Thus, intramuscular bioavailability of aluminum is necessarily 100%. It is all absorbed by the body, sooner or later.
By “not readily”, the CDC means that it is absorbed slowly. There have been two studies looking at the rate of aluminum absorption from ACVs: Flarend 1997 and Weisser 2019. Both tested both aluminum hydroxide (AH) and aluminum phosphate (AP) injected intramuscularly in animals. They measured blood concentration to estimate the speed of aluminum absorption.
At the rates observed by Flarend, assuming zero-order absorption, AP vaccines take 56 days to be fully absorbed and AH vaccines take 165 days. At the rates observed by Weisser, AP vaccines take 94 days to be fully absorbed and AH vaccines take 369 days. Based on the absorption rates observed by Flarend, all aluminum from infant ACVs is fully absorbed by one year of age.
Is uptake of aluminum from vaccines “low” because of the slow absorption?
We can use the results of Flarend on vaccine absorption to calculate daily aluminum uptake from vaccines throughout infancy. In the chart below, daily uptake of aluminum from vaccines is plotted against uptake from diet and air. The input scenario is full vaccination on the CDC schedule, typical formula-feeding and average urban air quality.
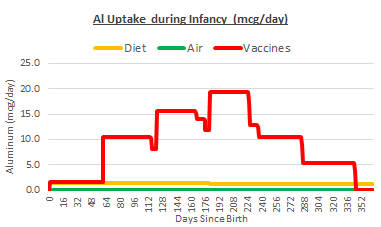
The slow absorption of the aluminum in vaccines does not alter the fact that vaccines are the biggest source of aluminum exposure in infants. At the peak, just after the 6 month shots, infants are exposed to nearly 20 mcg per day. This is the amount entering their bloodstream from each of their vaccine injection sites, like a nicotine patch. Most infants will never be exposed to more than 2 mcg per day from diet and air.
Since vaccines are the primary source of aluminum exposure to infants, it cannot be claimed that the amount of aluminum exposure is “low”.
Perhaps “low” is meant as compared to a safety level. Unfortunately, there are no uptake safety levels for chronic exposure to aluminum.
There are safety levels for oral intake. These have been derived from observations in animal experiments. They are the highest dose of aluminum for which no adverse effects were observed, converted to a Human-Equivalent Dose (HED). The US ATSDR set an oral intake Minimal Risk Level (MRL) of 1 mg/kgbw/day. The WHO JECFA set an oral intake safety level of 0.3 mg/kgbw/day.
These oral intake safety levels can be converted into a uptake safety levels by multiplying them by an assumed gut bioavailability of 0.3%. The units of mcg/kgbw can be converted into mcg using CDC average infant weight charts. The two derived uptake safety levels can then be added to our daily uptake chart:
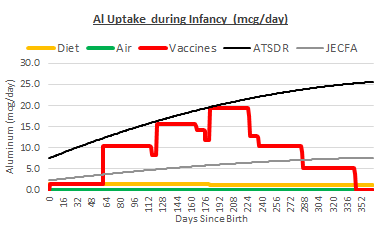
Uptake from vaccines alone far exceeds the safety level from the JECFA, and gets very close to the safety level from ATSDR. The cumulative impact of vaccines, diet, and air does exceed the ATSDR safety level for most infants. This is especially true of underweight infants and those on a high-aluminum diet.
The CDC ends it’s paragraph of information about aluminum adjuvant safety with this:
Read the research on aluminum exposure and vaccines. Also, see FDA’s web page on common ingredients in U.S. licensed vaccines for more information.
The first link is to a 2011 paper by Robert Mitkus and four of his colleagues at the US FDA. The paper describes a pharmacokinetic model developed for assessing the safety of ACVs in infants. The second link is to a webpage of the FDA, which refers only to the same Mitkus 2011 paper.
The CDC claims that aluminum adjuvant use is safe. Since Mitkus 2011 is the only scientific paper cited by the CDC in regard to aluminum in vaccines, this is presumably the strongest paper they can cite to support their claim. If this paper is shown to have serious flaws, it would be a crippling blow to the scientific case for vaccine adjuvant safety.
I have studied the Mitkus paper in great depth, along with the papers cited therein. I have reconstructed his whole model. I have expanded it to be able to cover more scenarios, i.e. variations in diet, air quality, vaccine schedules, etc. By doing that, I have identified many weaknesses, faulty assumptions, and outright errors.
I will explain the many flaws in the Mitkus model in future posts. In the meantime, here is an infographic explaining the most outrageous error. Mitkus used the wrong retention equation in his model!

The only scientific paper cited by the CDC in defense of their claim that aluminum adjuvants are safe contains this outrageous error, and there are many more.
There are good reasons to be concerned about aluminum adjuvant safety. Aluminum is a neurotoxin that can subtly alter neurodevelopment. It’s use in vaccines has increased over the years, in line with the increase in neurodevelopmental disorders.
The CDC is incorrect in describing the amount of aluminum in vaccines as “small” or total exposure from vaccines as “low”. Vaccines are in fact the primary source of aluminum exposure in infants. The CDC should update their website to remove these inaccurate descriptions.
Aluminum exposure from the vaccines on the CDC’s infant schedule exceeds the safety level established by the WHO. This calls for a reappraisal of the vaccine schedule: delaying or avoiding ACVs to reduce the risk of adverse neurodevelopmental effects from vaccines.
The CDC’s claim that aluminum exposure from vaccines is low enough to be considered safe relies on one single paper: a deeply flawed study by the FDA, presenting a pharmacokinetic model with many serious errors.
The CDC has changed the title of their webpage giving information about vaccines and autism from “Vaccines Do Not Cause Autism” to simply “Autism and Vaccines”.
Why did they change it? It could be due to the legal action of ICAN, or it could be an innocent “reformatting” of the website, as CDC defenders are claiming.
As both sides acknowledge, the text of the page is unchanged and still says that “vaccines do not cause ASD”, and that “there is no link between receiving vaccines and developing ASD” and “no links have been found between any vaccine ingredients and ASD”.
ICAN intend to pressure the CDC to remove these statements, too, because they say they are not supported by the scientific evidence.
Let us assess whether ICAN are right by looking at each of the sources cited by the CDC on this page to support their claim that vaccines do not cause autism.
Under the heading “There is no link between vaccines and autism”, the CDC writes that:
Some people have had concerns that ASD might be linked to the vaccines children receive, but studies have shown that there is no link between receiving vaccines and developing ASD. In 2011, an Institute of Medicine (IOM) report on eight vaccines given to children and adults found that with rare exceptions, these vaccines are very safe.
That Institute of Medicine (IOM) report was called “Adverse Effects of Vaccines: Evidence and Causality” (2012) and is a systematic review of all the scientific literature looking adverse effects of vaccines. The eight vaccines included in the study were MMR, Varicella, Influenza, Hepatitis A, Hepatitis B, HPV, DTaP, and Meningococcal. An impressive 76 different health outcomes were included in the study, one of which was autism.

However, only two vaccines – MMR and DTaP – were even examined in relation to the autism health outcome. Presumably, this is because there are no studies to examine. This alone makes the report insufficient evidence to claim that vaccines don’t cause autism. For all but two vaccines, according to the IOM, there have been no studies looking at associations to autism.
Regarding DTaP, the IOM concluded that:
The evidence is inadequate to accept or reject a causal relationship between DTaP and autism.
Their epidemiological assessment found the evidence “insufficient” (just one single study, rejected due to being based on data from a passive reporting system). Their mechanistic assessment found the evidence “lacking” (no studies at all).
The MMR is the only vaccine where the IOM made a conclusive statement:
The evidence favors rejection of a causal relationship between MMR and autism.
At best, therefore, this report supports the claim that the MMR does not cause autism. It cannot possibly support the bigger claim that vaccines do not cause autism.
In their epidemiological assessment, the IOM reviewed an impressive 22 studies looking for an association between MMR and autism. 12 of them were dismissed for being based on data from a passive surveillance system lacking an unvaccinated comparison population, or for being an ecological comparison study lacking individual-level data. A further 5 were dismissed as having “very serious methodological limitations”.
In their mechanistic assessment, the IOM reviewed 6 studies, but dismissed them all for not providing evidence beyond temporality, concluding that the mechanistic evidence is “lacking” when it comes to assessing a causal association between MMR and autism.
This left just 5 epidemiological studies that the IOM considered good enough to be used to conclude the lack of a causal association between MMR and autism. These were the studies by Taylor 1999, Farrington 2001, Madsen 2002, Smeeth 2004, and Mrozek-Budzyn 2010. The Mrozek-Budzyn study was acknowledged by the IOM as having “serious limitations”, and the Farrington study is based on the same data as the Taylor study.
I will review each of these MMR-autism studies in a future post.
The CDC writes that:
A 2013 CDC study added to the research showing that vaccines do not cause ASD. The study looked at the number of antigens (substances in vaccines that cause the body’s immune system to produce disease-fighting antibodies) from vaccines during the first two years of life. The results showed that the total amount of antigen from vaccines received was the same between children with ASD and those that did not have ASD.
The De Stefano study is a helpful addition to the research, in that it looks at the cumulative effects of multiple vaccines, rather than at vaccines in isolation as in the IOM report.
However, the study is deeply flawed, because no one who claims that vaccines cause autism says that it should be related to the number of antigens received. It is the other ingredients of vaccines that are of greater concern, especially the aluminum salts used as adjuvants. Nevertheless, grouping by number of antigens has the potential to act as a proxy for distinguishing fully vaccinated, partially vaccinated, and unvaccinated, so it may not be an entirely worthless measurement.
Unfortunately, the results in the study are skewed by the presence of three vaccines with 3000 antigens (DTP, DTP-Hib, and Typhoid), and then a large drop down to vaccines with 69 antigens (Varicella), 24 antigens (MMR), and all other vaccines having less than 15 antigens. Total number of antigens is therefore merely a proxy for number of doses of high-antigenic vaccines. From the chart below it can be seen that most subjects had either zero, three, or four doses of these high-antigenic vaccines, and this alone determines the groups used in the analysis.
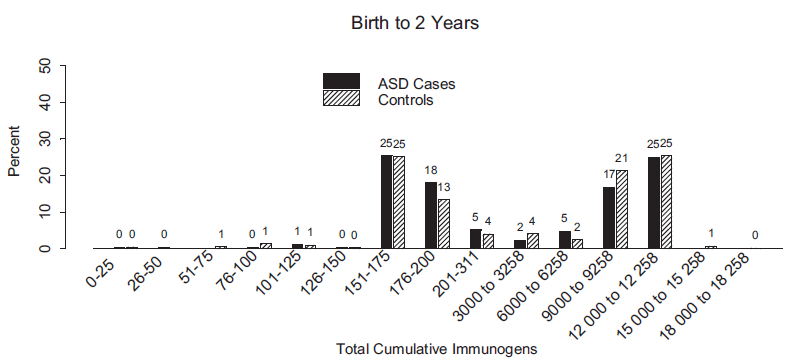
The chart also shows that there were no unvaccinated subjects in the study. Nobody received less than 50 antigens. The group that received zero high-antigenic vaccines received many other vaccines, because most of them had between 151 and 311 antigens. During the study period, the high-antigenic DTP vaccines were replaced by low-antigentic DTaP vaccines, so most of those in the “low antigens” group were fully vaccinated, just like most of the subjects in all the other groups.
At best, this study can be used to support the claim that high-antigenic vaccines do not cause autism any more than low-antigenic vaccines do. Since there are no high-antigenic vaccines used anymore, this is a moot conclusion. This study certainly cannot be used to support the claim that vaccines do not cause autism, because there were no unvaccinated subjects in the study.
The CDC then has a heading of “Vaccine ingredients do not cause autism” and writes that:
One vaccine ingredient that has been studied specifically is thimerosal, a mercury-based preservative used to prevent contamination of multidose vials of vaccines. Research shows that thimerosal does not cause ASD. In fact, a 2004 scientific review by the IOM concluded that “the evidence favors rejection of a causal relationship between thimerosal–containing vaccines and autism.” Since 2003, there have been nine CDC-funded or conducted studies that have found no link between thimerosal-containing vaccines and ASD, as well as no link between the measles, mumps, and rubella (MMR) vaccine and ASD in children.
That Institute of Medicine (IOM) report was called “Immunization Safety Review: Vaccines and Autism” (2004) and is a systematic review of all the epidemiology studies looking at associations between vaccines and autism. The report only examines studies of thimerosal-containing vaccines (TCVs) and the MMR vaccine (superceded by their 2012 review).
The IOM reviewed 12 studies looking for an association between TCVs and autism. 6 of them were dismissed for being based on data from a passive surveillance system lacking an unvaccinated comparison population, or for being an ecological comparison study lacking individual-level data. One study based on Vaccine Safety Datalink (VSD) data was dismissed as “uninterpretable”.
This left 5 studies on which the IOM relied for their conclusion that TCVs do not cause autism: Hviid 2003, Miller 2004, Verstraeten 2003, Madsen 2003, Stehr-Green 2003.
The CDC cites a two-page PDF that lists and briefly summarises eight further studies to support their claim TCVs do not cause autism: Barile 2011, Price 2010, Tozzi 2009, DeStefano 2009, McMahon 2008, Thompson 2007, Verstraeten 2003, Stehr-Green 2003 (included twice).
I will review each of these TCV-autism studies in a future post.
The CDC writes that:
Between 1999 and 2001, thimerosal was removed or reduced to trace amounts in all childhood vaccines except for some flu vaccines. This was done as part of a broader national effort to reduce all types of mercury exposure in children before studies were conducted that determined that thimerosal was not harmful. It was done as a precaution. Currently, the only childhood vaccines that contain thimerosal are flu vaccines packaged in multidose vials. Thimerosal-free alternatives are also available for flu vaccine.
Thus, the question of whether TCVs cause autism is now only of historical interest within the wider context of the question of whether vaccines cause autism. The studies cited to support that the claim that TCVs do not cause autism have significant weaknesses, and there is considerable evidence suggesting that TCVs do cause autism.
Since they are now rarely used, TCVs are clearly not an ingredient of concern to those who claim that vaccines cause autism today. Therefore, whether TCVs do cause autism is a moot point in respect of this present-tense claim.
Besides thimerosal, no other vaccine ingredients are named. All the ingredients in vaccines today are addressed by the CDC with a single sentence:
Besides thimerosal, some people have had concerns about other vaccine ingredients in relation to ASD as well. However, no links have been found between any vaccine ingredients and ASD.
No studies at all are cited to support this claim. The link goes to a page merely listing types of vaccine ingredients and their purpose:
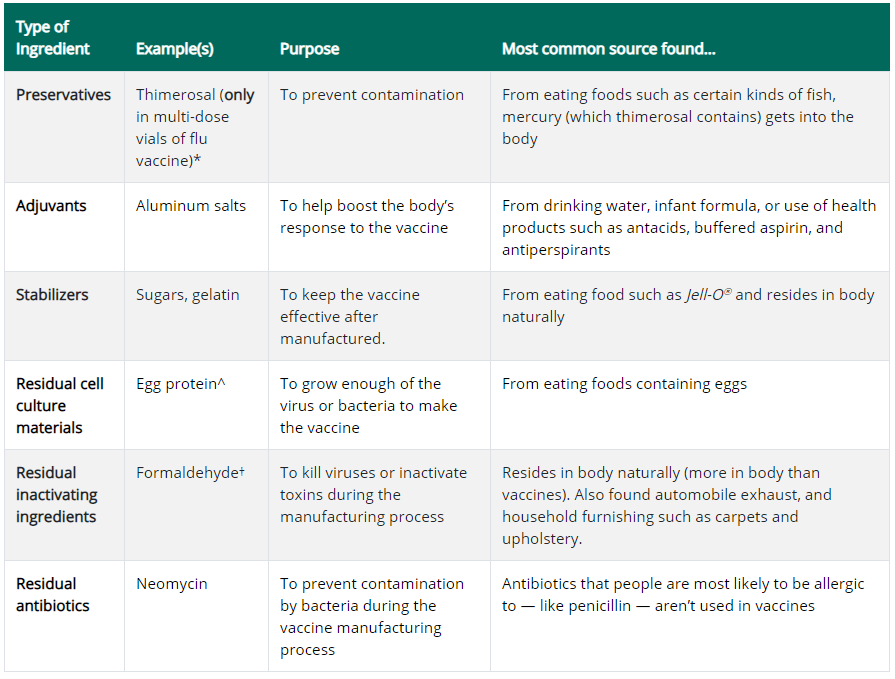
Aluminum salts are the vaccine ingredient of biggest concern when it comes to autism, because aluminum is a neurotoxin that has been observed to induce subtle neurological effects and autism-like behaviour in animals exposed to it. The toxic nature of aluminum is why aluminum salt works as a vaccine adjuvant, ensuring that the activation of the immune system is strong enough to result in immunological memory.
There have also been health concerns raised about formaldehyde, glutamate, polysorbate-80, antibiotics, animal cells and fetal cells.
No studies are cited on this page to support the claim that vaccine ingredients do not cause autism.
The CDC provides a list of five links:
Facts About Autism Spectrum Disorders
Fact Sheet: Understanding Thimerosal, Mercury, and Vaccine Safety
IOM Report: Adverse Effects of Vaccines: Evidence and Causality, 2011
The first link has information about autism but not about vaccines. The second, fourth and fifth links are about TCVs. The third link is to the IOM 2011 Report cited above.
The CDC provides a list of seven links:
Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: An evidence-based meta-analysis of case-control and cohort studiesexternal iconexternal icon. Vaccine. 2014 June;32(29):3623–3629.
Schechter R, Grether JK. Continuing increases in autism reported to California’s developmental services system: Mercury in retrogradeexternal icon. Arch Gen Psychiatry. 2008;65:19-24.
Institute of Medicine. Immunization Safety Review. Vaccines and Autismexternal icon Board of Health Promotion and Disease Prevention, Institute of Medicine (National Academy Press, Washington, DC, 2004).
Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Association between thimerosal-containing vaccine and autism pdf icon[PDF – 5 pages]external icon. JAMA. 2003;290:1763–6.
Madsen KM, Hviid A, Vestergaard M, Schendel D, Wohlfahrt J, et al. A population-based study of measles, mumps, and rubella vaccination and autismexternal icon. N Engl J Med. 2002;347 (19):1477–1482.
Ball L, Ball R, Pratt RD.An assessment of thimerosal in childhood vaccinesexternal icon. Pediatrics. 2001;107:1147–1154.
Joint statement of the American Academy of Pediatrics (AAP) and the United States Public Health Service (USPHS)external icon. Pediatrics. 1999;104:568–9.
The first link is to a meta-analysis that combined the results of five MMR-autism studies, and five TCV-autism studies, all of which individually found no associations. Most of the studies in this Taylor 2014 meta-analysis are the same papers reviewed by the IOM, cited above (including some they rejected for having serious methodological limitations); there is one new TCV study (Andrews 2004) and one new MMR study (Uno 2012).
The second link is to an ecological study, Schechter 2008, showing the removal of TCVs did not coincide with any reduction of cases of autism in California. The third link is to the IOM 2004 Report cited above. The fourth link is to the Hviid 2003 study of TCVs-autism. The fifth link is to the Madsen 2002 study of MMR-autism, both in the IOM Reports cited above. The sixth link is to an outdated review of TCVs that does not reference autism. The seventh link is to a statement by the AAP regarding removing thimerosal from vaccines.
There is no evidence cited on the CDC’s “Autism and Vaccines” page to support their claim that vaccines do not cause autism. The only evidence cited relates either to one single vaccine: the MMR, or to one single vaccine ingredient: thimerosal. Even if this evidence is accepted, it does not follow that vaccines do not cause autism.
More studies are needed in order to determine whether vaccines cause autism, starting with the most basic kind: epidemiological studies that compare health outcomes in vaccinated and unvaccinated children.
Interestingly, this was pointed out by the IOM itself in their 2013 report “The Childhood Immunization Schedule and Safety”:

Without any studies looking at autism as a health outcome and comparing vaccinated and unvaccinated groups, there is no support for the CDC’s claim that vaccines do not cause autism. They were right to change the title of their page. They now need to correct the rest of it.
This infographic presents some of what we know about immune activation events causing autism and states the vaccine-autism hypothesis.
Given the close association between the immune system activations and autism, supported by animal testing, the vaccine-autism hypothesis is highly plausible.
After all, the purpose of vaccines is to activate the immune system, which is something we know can trigger autism.
Sources are listed and linked below, and I have copied a line from the abstract of each study for the convenience of the reader.
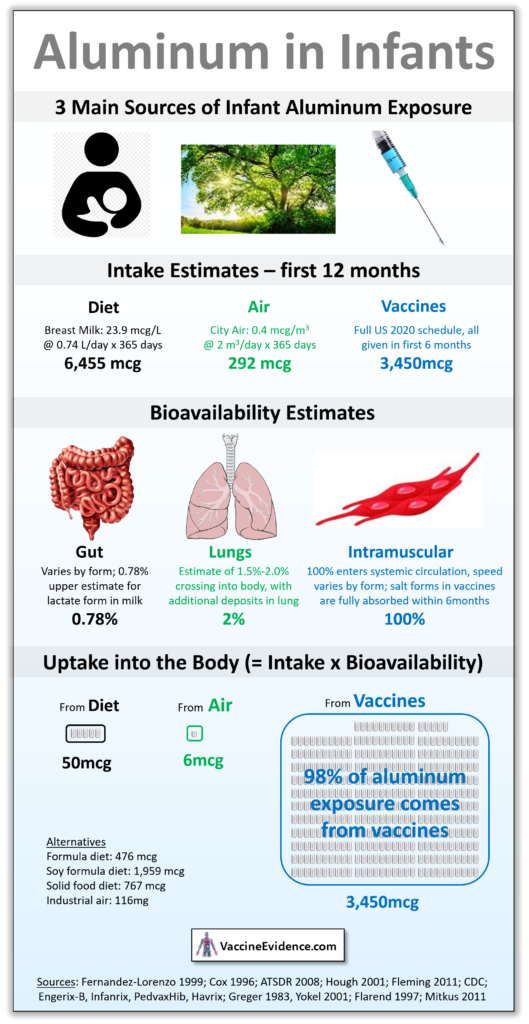
This infographic demonstrates that the majority of exposure to aluminum in infants comes from vaccines.
Children fully vaccinated using the CDC schedule for the first 6 months will receive 3,450mcg of aluminum into the blood from those vaccines, as the aluminum salts used as vaccine adjuvants slowly dissolve at the intramuscular injection site and enter the bloodstream. This process takes no more than 6 months, so 100% will have been absorbed by 1 year of age.
Breast milk only contains an average of 23.9 mcg/L, and infants drink an average of 0.74 L/day, for a total dietary intake of 6,455mcg of aluminum in the first 12 months. Most of this will be defecated out, but up to 0.78% could leak across the gut lining and enter the bloodstream. This would amount to 50mcg of aluminum over the year. Formula milk contains an average of 226 mcg/L and soy formula can contain up to 930 mcg/L. With the same amount drunk and same gut bioavailability, this would be 476mcg and 1959mcg respectively entering the gut. Infants on a semi-solid food diet will be getting an average of 767mcg. There is no diet that exposes infants to more aluminum through the gut than they are from vaccines.
The third biggest normal exposure is air, but the amounts here are insignificant. Multiplying average tidal volume by average respiratory rate results in 730 m3 of air being breathed by an infant by 1 year of age. Normal city air has around 0.4 mcg/m3 of aluminum, resulting in an intake of 292mcg. Lung bioavailability has been estimated at 2%, so only 6mcg of aluminum enters the bloodstream through the lungs during infancy. Even in a city with high pollution or industrial air, with up to 8 mcg/m3 of aluminum, that uptake figure only reaches 116mcg. Infant exposure to aluminum via the air is insignificant.
Sources for all these figures are listed and linked below.
Sources: